cobas® infinity
How to do more with less when it comes to operational excellence in the clinical laboratory?
Digital solutions for the laboratory and beyond
The medical diagnostics sector receives less than 2% of the overall healthcare budget, but at the same time the data that comes from the lab impacts 60% to 70% of all clinical decisions.
Our clients talk about the benefits obtained from implementing infinity
Roche offers a wide range of digital solutions designed to meet your current and future needs, based on the following principles:
Salud Digna, Mexico
"... with cobas® infinity it is possible to centralize the operation of the laboratory, have real-time visibility of the status of the tests, and thereby support its objectives of achieving high levels of efficiency..."
It is essential that the microbiology area is fully integrated into the clinical laboratory workflow. Due to their complexity, in most cases the samples are processed manually; this involves keeping detailed records, which is time consuming and increases the risk of human error. Having identified this situation, clinical laboratories have implemented the cobas infinity microbiology module to mitigate the potential for errors and delays associated with a manual workflow.
Referencia Laboratorio Clinico, Dominican Republic
"... almost a year after having implemented cobas® infinity, they managed to reduce by 40% the time it takes to obtain positive reports in the area of microbiology..."
Lean Validation
One of the main challenges in the clinical laboratory is the validation of results, which is why laboratories of different sizes implement automatic validation through cobas® infinity and use co-creation processes with Roche to optimize your processes, guarantee the quality of your results and dedicate time to other tasks that generate more value.
Referencia Laboratorio Clinico, Dominican Republic
"... the implementation of cobas® infinity Lean Validation for the core processing of the sample and more than a year after its implementation, managed to reduce by 50% the time it takes to validate results..."
Production Monitoring
The lab manager can proactively monitor the entire operation and maximize productivity

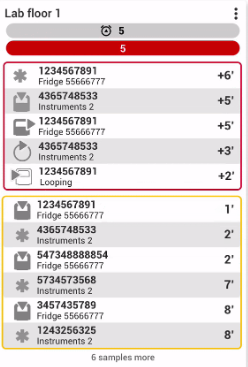

Receive real-time notifications of instrument connections, and order interruptions coming from the LIS/HIS.
Ensure the continuity of the operation.
Monitor potentially late samples, receive notifications and take preventive action.
Track workload and make decisions on the fly to ensure response times.
Contact
We can help you maximize your operational efficiency.
Leave us your details and a Specialized Consultant will contact you shortly.
References
1. European IVD Market Statistics. EDMA. 2013.
2. Rohr U-P, Binder C, Dieterle T, Giusti F, Messina CGM, Toerien E, et ál. (2016) The Value of In Vitro Diagnostic Testing in Medical Practice: A Status Report. PLoS ONE 11(3): e0149856. https://doi.org/10.1371/journal.pone.0149856.
Legal and Privacy Notice
This website contains product information that is intended for a broad range of audiences and may contain product details or information that would not otherwise be accessible, approved or otherwise not accessible, approved or valid in your country. Please note that we assume no responsibility or liability for accessing information that may not comply with any legal process, regulation, registration or use in the country of its origin. Please also note that the information on this web site should not be used to diagnose, treat, cure or prevent any disease without the advice of a qualified medical professional, and is not a substitute for medical advice or a medical examination.
MC--10518

